New Discovery on the Phosphorylation Regulation of Calcium-release Channels
Ion channels are important drug targets as well as pesticide targets. A research team led by Prof. Yuchi from School of Pharmaceutical Science and Technology discovered a new phosphorylation regulation mechanism on calcium channels. Their results have been published in the journal BMC Biology entitled Crystal structure of diamondback moth ryanodine receptor Repeat34 domain reveals insect-specific phosphorylation of sites. (Link to the article: https://bmcbiol.biomedcentral.com/articles/10.1186/s12915-019-0698-5).
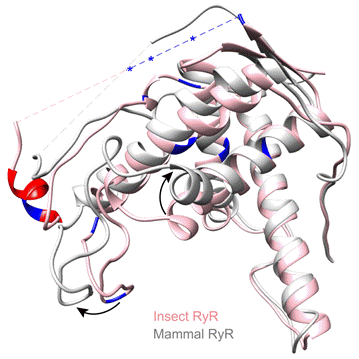
Ryanodine receptors (RyRs) are large calcium-release channels located in the sarcoplasmic reticulum membrane, which play a central role in the excitation-contraction coupling of muscles. Pesticides worth of billions of dollars take RyRs as their target and are sold worldwide every year. Professor Yuchi's team solved the high-resolution crystal structure of the important phosphorylation domain of insect RyR, revealing its significant structural difference from its mammalian counterpart. The research team used protein mass spectrometry to identify insect-specific phosphorylation sites which can be modulated in a temperature-dependent manner. This special property can help insects adapt to the ambient temperature change through converting the temperature signal into intracellular calcium signal. It was also found that insect RyR has a ligand-binding pocket conserved only in insects, which can be used as a potential binding site for new insecticides. The findings from this study lay a foundation for the further development of green pesticides with high species-specificity.
By: the School of Pharmaceutical Science and Technology
Editors: Eva Yin & Doris Harrington

