Systematically Reviews New Paradigms in Ultrasound-Driven Disease Theranostics
Recently, Professor Shi Zhiyuan’s team at School of Pharmaceutical Science and Technology, Faculty of Medicine, Tianjin University published a comprehensive review in Cell Biomaterials , detailing progress and future directions in ultrasound-enabled theranostics. The work systematically analyzes ultrasound’s mechanisms and applications in precision medicine through three dimensions (Figure. 1): Sono-physics, Sono-chemistry and Sono-biology.
While ultrasound is widely perceived as a gentle medical imaging tool (e.g., prenatal B-scan, cardiac color Doppler), it is undergoing a transformative shift: evolving from diagnostic imaging to an active therapeutic platform. Recent advances in materials science, nanotechnology, and molecular biology have revealed ultrasound’s capacity not only to visualize tissues but also to precisely influence cellular, molecular, and genetic behaviors. This establishes ultrasound as a critical bridge connecting physics, chemistry, and life sciences.

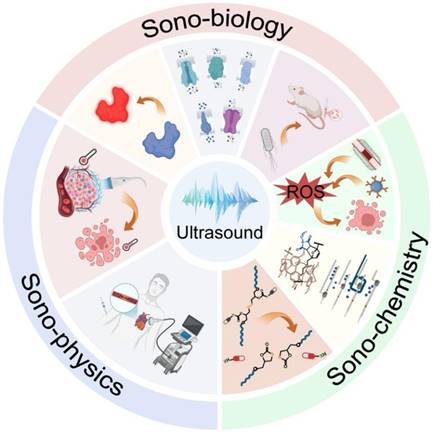
Figure. 1. Schematic of three ultrasound-driven theranostic approaches.
Unlike ionizing radiation (e.g., X-rays), ultrasound combines non-ionizing properties with deep tissue penetration (centimeters to tens of centimeters). Its frequency, intensity, and duration can be precisely tuned, offering triple advantages: safety, controllability, and penetration. Crucially, it enables non-invasive intervention at target sites without surgery or implants, laying the foundation for precision medicine.
Sono-physics: Acoustic Waves Meet Microbubbles and Tissues
The ultrasonic cavitation effect serves as the fundamental mechanism whereby acoustic pressure drives microbubbles in liquid to oscillate or collapse, thereby releasing mechanical energy. In medical applications, the introduction of engineered microbubbles enables precise modulation: under mild acoustic conditions, tissue permeability is enhanced to facilitate targeted drug delivery, while high-energy exposure induces tumor ablation via inertial cavitation. This capability is particularly transformative for overcoming the blood-brain barrier limitation, thereby pioneering novel therapeutic pathways for neurological disorders in a non-invasive and reversible manner.
Sono-chemistry: Activating Drugs and Materials with Ultrasound
Ultrasound activates sonosensitizers to generate reactive oxygen species (ROS), selectively destroying tumors/pathogens (sonodynamic therapy). It also triggers chemical bond cleavage (Figure. 2) for "on-demand" drug release (ultrasound-triggered drug delivery), significantly reducing systemic toxicity.
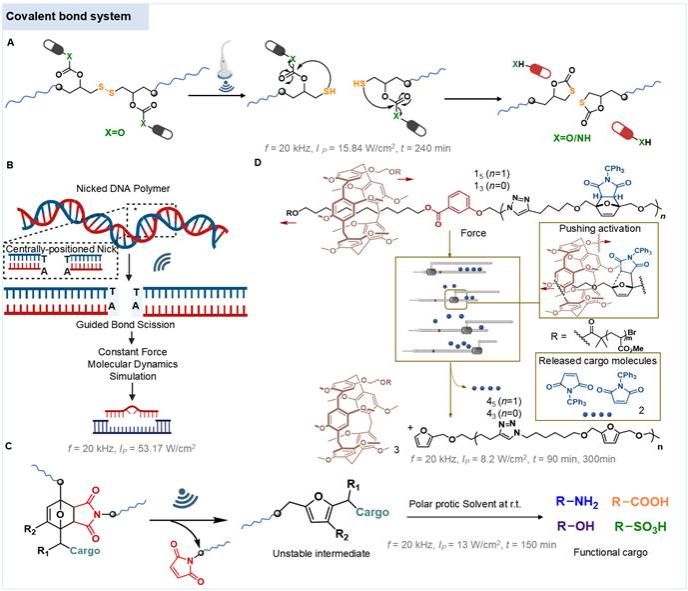
Figure. 2. Covalent bond cleavage for drug release.
Sono-biology: How Ultrasound Influences Cells and Nerves?
Focused on how biological systems perceive and respond to ultrasound, research reveals that mechanical forces activate mechanosensitive ion channels (Figure. 3), regulating calcium signaling to gene expression, particularly amplified in neural systems. Emerging sonogenetics techniques express mechanosensitive proteins in specific cells, enabling remote, non-invasive neural circuit modulation and offering new pathways for brain disease research.
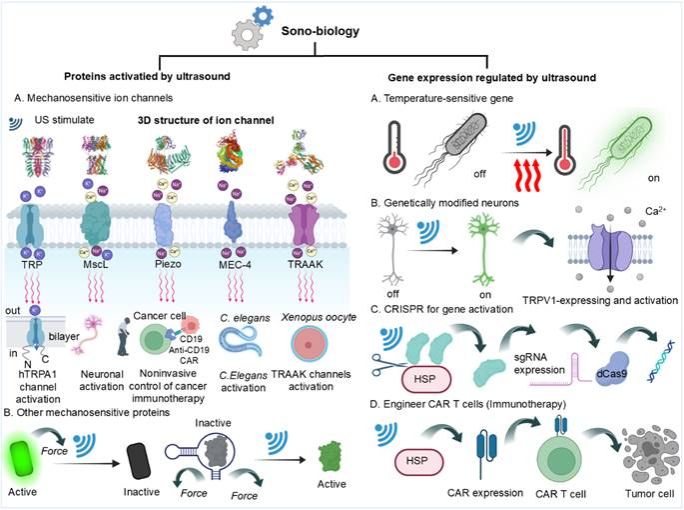
Figure. 3. Ultrasound-activated biological systems.
Challenges and Future Outlook
Clinical translation faces hurdles in material safety, mechanistic clarity, and real-time monitoring standardization, necessitating interdisciplinary collaboration (physics/chemistry/materials/biology/medicine). With the integration of AI, smart materials and molecular engineering, ultrasound is making an intelligent leap forward, accelerating the realization of personalized precision therapy.
This study, titled “Advancing Disease Theranostics with Ultrasound: Sono-physics, Sono-chemistry, and Sono-biology” was published in Cell Biomaterials with Tianjin University as the primary affiliation. Key contributors include Ph.D. student Jianing Zhang, Master’s students Xiaomiao Yu, Liutianyun Yuan, and Shang Jia.
By School of Pharmaceutical Science and Technology
Editor: Sun Xiaofang

