Research Team Achieves Breakthroughs in Hydrogen-Electric Conversion Materials
Recently, Professor Michael Guiver, Professor Yan Yin, and Professor Junfeng Zhang 's team from School of Mechanical Engineering at Tianjin University has made significant strides in core areas of hydrogen-electrical energy interconversion, including ion-exchange membranes, electrode interfaces, and catalyst engineering. By proposing a series of innovative material design concepts and engineering strategies, the team has effectively enhanced the performance and efficiency of hydrogen energy devices while extending their operational lifespan. Their recent findings have been published in prestigious international journals including Joule and Angewandte Chemie International Edition. As a key component of State Key Laboratory of Engines and National Industry-Education Platform for Energy Storage (Tianjin University), the research group is consistently dedicated to the research, development, and application of materials for hydrogen-electrical energy interconversion technologies. Their work focuses on novel, clean, and highly efficient new energy conversion technologies, contributing scientific and technological strength to achieving the Dual Carbon goals and advancing green energy development.
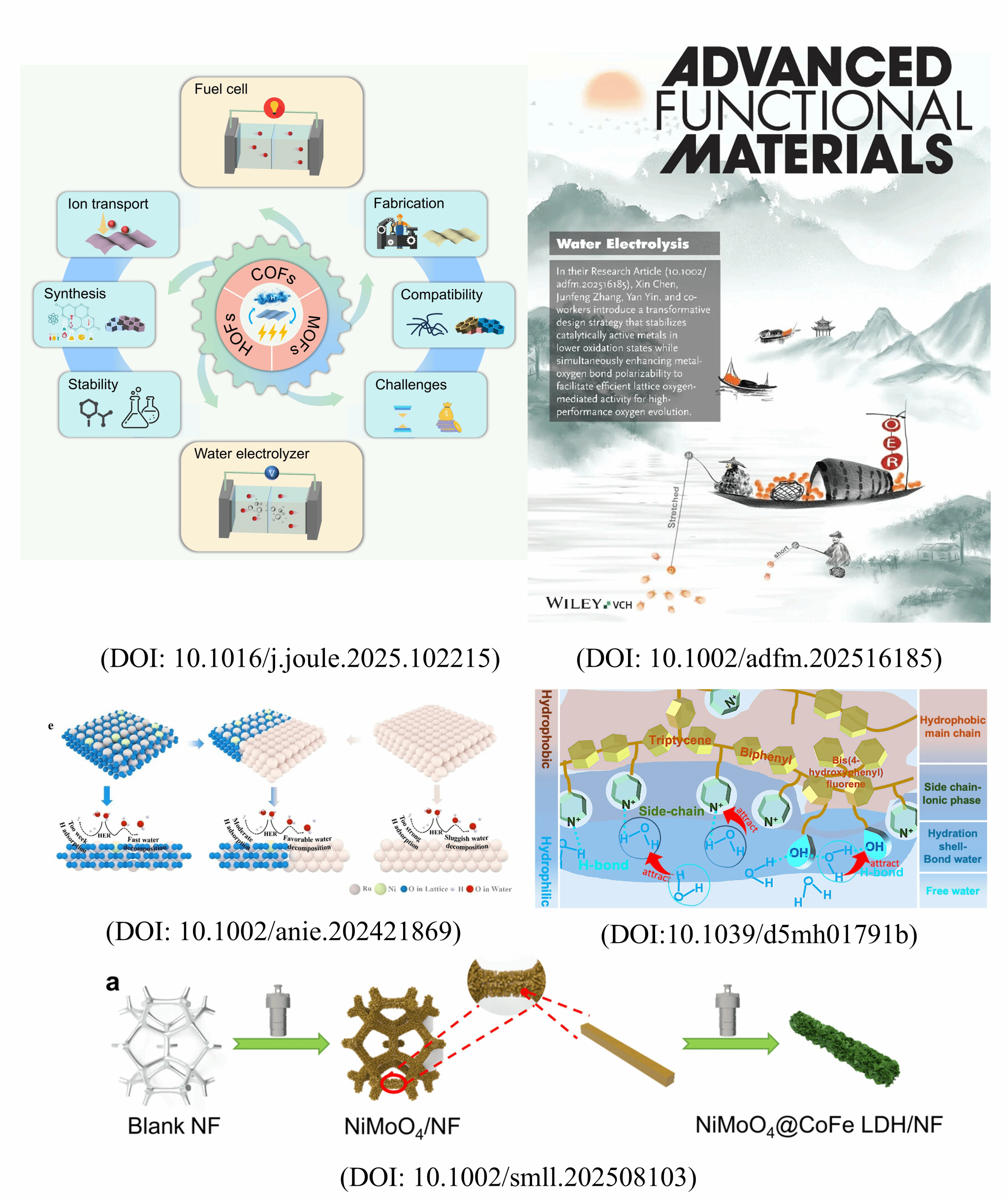
Overview of achievements
Ion-Exchange Membranes Based on Framework Materials
In the field of ion-exchange membranes, the team published a review article titled "Ion-exchange membranes based on framework materials for hydrogen‒electrical energy interconversion" in Joule (DOI: 10.1016/j.joule.2025.102215). This review systematically summarizes recent progress in porous framework materials (such as COFs, MOFs, and HOFs) for ion-exchange membranes. Conventional polymer-based ion-exchange membranes face challenges in practical applications, including chemical and mechanical degradation, sensitivity to moisture/temperature fluctuations, and high manufacturing costs. Porous framework materials, with their customizable structures, rich pore designs, and excellent dimensional stability, offer new solutions for regulating ion transport behavior and enhancing membrane performance. Notably, the pioneering research on Metal-organic frameworks (MOFs), a class of materials that were recognized by the 2025 Nobel Prize in Chemistry, aligns closely with the design concepts for porous framework materials discussed in this review. Through in-depth analysis of material synthesis, properties, performance, and existing challenges, the team provides valuable insights and strategic references for future research on ion-exchange membranes.
Decoupling Activity and Stability in OER Catalysts
In the area of oxygen evolution reaction (OER) catalysts, which are related to electrolyzers for hydrogen production, the team published a cover paper titled “Stretching metal-oxygen bonds to decouple activity and stability of water electrolysis” in Advanced Functional Materials (DOI: 10.1002/adfm.202516185, Frontispiece). This research breaks from the conventional pursuit of high-valence metal species. Instead, it innovatively employs a dual strategy of "stabilizing low-valence metals + stretching metal-oxygen bonds," successfully resolving the typical trade-off between catalyst activity and stability. By stabilizing metals like nickel and iron in lower valence states and elongating the metal-oxygen molecular bonds, the team effectively activated the reactivity of lattice oxygen while significantly enhancing structural stability. Experimental results demonstrated that the developed NiFe-LP catalyst, based on this strategy, requires a smaller overpotential of only 219 mV to achieve a current density of 10 mA cm⁻² and operates stably for over 1000 hours at a high current density of 5 A cm-2. This achievement provides a novel design approach for developing efficient, stable catalysts for water electrolysis, with significant importance for advancing green hydrogen technology.
Additionally, the team published a research paper in Small titled “NiMoO@CoFe-LDH anode design to improve oxygen bubble transport in dynamic water electrolysis” (DOI: 10.1002/smll.202508103). This work involved the synthesis of Co-Fe bimetallic oxyhydroxides via a hydrothermal reaction, resulting in a 35 cm-1 red shift of the M-OOH bond, which reduced the energy barrier of the OER rate-determining step by 0.28 eV. The mass activity reached 63.36 mA/mg at an overpotential of 300 mV, approximately 5.75 times that of RuO2, demonstrating a significant synergistic catalytic effect. Using high-speed camera technology, the team revealed for the first time the dynamic coupling mechanism between bubble evolution and electrode corrosion, offering a new solution for developing efficient electrolyzer anodes adaptable to the fluctuating nature of renewable power sources like solar and wind.
Breakthrough in HER Catalysts at Ampere-Level Currents
Substantial progress has also been made in hydrogen evolution reaction (HER) catalysts. The researchers published a paper titled “Modulating built-in electric field strength in Ru/RuO2 interfaces through Ni doping to enhance hydrogen conversion at ampere-level current” in Angewandte Chemie International Edition (DOI: 10.1002/anie.202421869, HOT Paper). Combining density functional theory calculations with experimental validation, the study developed a Ni-doped RuO2 catalyst. By modulating the built-in electric field at the interface with Ru, the catalyst optimizes the interfacial electronic structure and the Gibbs free energy of H adsorption, thereby significantly enhancing HER performance. This Ni-RuO2 catalyst requires an overpotential of only 134 mV to reach a current density of 1 A cm-2 and maintains stable operation for over 1000 hours at 60 °C. This research, utilizing heteroatom doping to precisely construct and tune the interfacial BIEF, provides an effective strategy for designing efficient alkaline HER electrocatalysts capable of operating at ampere-level current densities.
Addressing Water Management in AEM Water Electrolysis
Addressing the critical challenge of water management under "dry cathode" conditions in anion exchange membrane water electrolysis, the team published a paper in Materials Horizons titled “Microphase water control utilizing highly hydrophilic anion-exchange ionomers” (DOI: 10.1039/d5mh01791b). They proposed a novel three-dimensional, highly hydrophilic ionomer material named Pip(BPF-BPN)/Trip-x. By incorporating a sterically rigid backbone and phenolic hydroxyl side chains, the material creates abundant free volume and a hydrogen-bond network, significantly enhancing both hydrophilicity and structural stability. Experimental results show that this ionomer outperforms commercial materials in AEMWE tests, offering a promising new pathway for next-generation green hydrogen production technology.
Editor: Eva Yin

